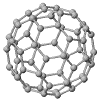
 Responding to an earlier post on inert gases, a commenter wondered if buckminsterfullerene might act as an inhalation anesthetic - given that, like xenon, it's a large, polarizable ball of electron density. It might, if you could get enough to inhale. At room temperature, the vapor pressure is 5 x 10-6 torr. Very roughly, that's about a billionth of atmospheric pressure. For comparison's sake, the pressure of xenon necessary to induce anesthesia is about 500 torr, or 65% of normal atmospheric pressure. If you want higher pressures, you need higher temperatures: buckminsterfullerene sublimes (goes directly from the solid to the gas phase, like dry ice) just above 1000
Responding to an earlier post on inert gases, a commenter wondered if buckminsterfullerene might act as an inhalation anesthetic - given that, like xenon, it's a large, polarizable ball of electron density. It might, if you could get enough to inhale. At room temperature, the vapor pressure is 5 x 10-6 torr. Very roughly, that's about a billionth of atmospheric pressure. For comparison's sake, the pressure of xenon necessary to induce anesthesia is about 500 torr, or 65% of normal atmospheric pressure. If you want higher pressures, you need higher temperatures: buckminsterfullerene sublimes (goes directly from the solid to the gas phase, like dry ice) just above 1000While likely impractical as an anesthetic, buckminsterfullerene has asthetic properties. It's a highly symmetric molecule - having iscosohedral symmetry. Kroto and Smalley discovered the new allotrope of carbon, C60, in vaporized graphite and named it for the architect (Buckminster Fuller) who made famous the geodesic domes it resembled. Two more familiar allotropes of carbon are graphite and diamond.
Allotropes are differing forms of the same element. The roots of the word are Greek - allos for different and tropos for "turn of mind". A different turn of mind? It's what Smalley needed to propose the now iconic structure, over a beer at his kitchen table.
Another allotrope of carbon is lonsdaleite - named for Kathleen Lonsdale, an Irish crystallographer who determined the structure of benzene and my brother-in-law's godmother.
















C60 as intravenous anesthetic solubilized and transported to nerve sheath expansion inside a large hydroxypropylated cyclodextrins or Rebek's modular self-assembling glycolurils,
ReplyDeleteAngew. Chem. (Int. Ed.) 41(9) 1488 (2002)
Cessation of anesthesia is left as an execise for the alert reader.
There is one more accessible allotrope of carbon: polycarbyne, (-C#C-)n. Roald Hoffmann proposed an elegant modeled carbon allotrope. A parallel plane of vertical polyacetylenes is bonded top and bottom by parallel planes of of vertical polyacetylenes running at right angles, and so on.
That is an idea, though how you would dump it out at the nerve sheath is a pretty problem, and as you point out, given it's insolubility, hard to imagine how the body would clear it.
ReplyDeleteMy general chem class and I had discussed allotropes and bucky balls when you made the earlier comment - which gave me an incentive to write about it. Thanks!
There are a bunch of lovely architecturally appealing allotropes of carbon...polycarbyne among them.
Organic chemistry is flat. Molecules are planar graphs with no intersecting bonds. That allows systematic skeletal nomenclature, e.g., Schlegel diagrams (on the right) and for C60.
ReplyDeleteC27H28, smaller than cholesterol and with only single bonds, will not stretch and flatten without intersecting bonds. NIST rewrote its commercial stereochemistry software ~2003. How much fun is causing that!
Do chemistry, be naughty.
Not every organic molecule has a planar graph in which no bond crosses another. Mobius ladder molecules, of which there is at least one synthesized example do not have such a graph.
ReplyDeleteNon-orientable surfaces can be... disturbing, maths vs. rendered models.
ReplyDeleteDraw a chiral center (scalene triangles are 2-D chiral) on a transparent Möbius strip. Make a strap from a folded piece of overhead projector film and duplicate the chiral image. Slide the image around and finally back to its starting point without leaving the surface. It is inverted! Where did the reflection take place? You could have stretched the image between your fingers so it was always planar and never twisted as the Möbius strip translated by.
Look at the generating equations of a Möbius strip. Do you get a different result for reversing the signs of all x or all y or all z, or all x and y and z? Is the twist chiral? Are physical models chiral?
Do a Möbius strip's mathematical and physical model possess non-planar skeletal graphs by Kuratowski's theorem?
Fun!
How did Lonsdale determine your brother-in-law's godmother? ;-)
ReplyDeletealso carbon nanotubes, graphene, and carbon aerogel
ReplyDelete