Slate magazine
notes that occasional cases of cholera have been reported in the Gulf states, suggesting that the bacteria
Vibrio cholerae is alive and well in that area. Recently, a group at Universtiy of Washington [O'Neal, Claire J., Jobling, Michael G., Holmes, Randall K., Hol, Wim G. J.
"Structural Basis for the Activation of Cholera Toxin by Human ARF6-GTP"
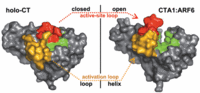
Science 2005 309: 1093-1096]has used x-ray crystallography to probe how the cholera toxin provokes such massive diarrhea when it takes up residence in the human gut. A figure from the paper shows how the overall 3-dimensional structure of the toxin changes when an activator protein binds to it. Protein chemists use the term "allosteric" to describe these mechanisms.
The picture is what interests me. I lectured on Monday about the probability density that you can compute using quantum mechanics (ψ*ψdτ). You can also measure the density experimentally using x-ray techniques, as Hol's group did for the cholera toxin. These sorts of pictures don't actually show the full density function, but a surface of constant electron density, usually around .002 e/bohr
3, which seems small. A bohr is very small (0.529 x 10
-10 )meters, so this corresponds to about one mole of electrons (on the order of 10
23!) per cubic inch. This sounds like a lot, until you realize it's about the same as the number of electrons in a teaspoon of water!