James Clerk Maxwell was a Scottish mathematician and physicist. He is perhaps most famous for his extension and refinement of Faraday's equations describing magnetic and electric fields. He reduced the necessary set of equations to four simple partial differential equations, the eponymous Maxwell equations, publishing the work in 1873.
He also worked in thermodynamics, lending his name to another set of four key differential relationships (the Maxwell relations). He also independently derived the Boltzmann distribution of the kinetic energies of gas molecules. Maxwell's demon, a "finite being" who opened a door between to a box of molecules, letting them in and out depending on the amount of energy they had was a rhetorical device Maxwell used to show that entropy and heat flow were, at their core, statistical phenomena.
Lord Kelvin (aka William Thomson) applied the demon tag, Maxwell used the term "finite being"!
- Home
- Angry by Choice
- Catalogue of Organisms
- Chinleana
- Doc Madhattan
- Games with Words
- Genomics, Medicine, and Pseudoscience
- History of Geology
- Moss Plants and More
- Pleiotropy
- Plektix
- RRResearch
- Skeptic Wonder
- The Culture of Chemistry
- The Curious Wavefunction
- The Phytophactor
- The View from a Microbiologist
- Variety of Life
Field of Science
-
-
Change of address6 months ago in Variety of Life
-
Change of address6 months ago in Catalogue of Organisms
-
-
Earth Day: Pogo and our responsibility9 months ago in Doc Madhattan
-
What I Read 20249 months ago in Angry by Choice
-
I've moved to Substack. Come join me there.11 months ago in Genomics, Medicine, and Pseudoscience
-
-
-
-
Histological Evidence of Trauma in Dicynodont Tusks7 years ago in Chinleana
-
Posted: July 21, 2018 at 03:03PM7 years ago in Field Notes
-
Why doesn't all the GTA get taken up?7 years ago in RRResearch
-
-
Harnessing innate immunity to cure HIV9 years ago in Rule of 6ix
-
-
-
-
-
-
post doc job opportunity on ribosome biochemistry!10 years ago in Protein Evolution and Other Musings
-
Blogging Microbes- Communicating Microbiology to Netizens11 years ago in Memoirs of a Defective Brain
-
Re-Blog: June Was 6th Warmest Globally11 years ago in The View from a Microbiologist
-
-
-
The Lure of the Obscure? Guest Post by Frank Stahl13 years ago in Sex, Genes & Evolution
-
-
Lab Rat Moving House14 years ago in Life of a Lab Rat
-
Goodbye FoS, thanks for all the laughs14 years ago in Disease Prone
-
-
Slideshow of NASA's Stardust-NExT Mission Comet Tempel 1 Flyby14 years ago in The Large Picture Blog
-
in The Biology Files
The Who, What, When, Where and Why of Chemistry
Chemistry is not a world unto itself. It is woven firmly into the fabric of the rest of the world, and various fields, from literature to archeology, thread their way through the chemist's text.
Made from sugar, so it tastes like sugar
I was reading the South Beach Diet book the other night (never mind why!) and noticed that the author recommends (without giving an explicit brand name) an artificial sweetener derived from sugar. A friend on the diet will only use sucralose, saying that the only one that "works right" with the diet. (The SB book itself says it doesn't matter, it's just personal preference.) A recent ad campaign claims, "Made from sugar so it tastes like sugar". As a chemist, I read this and cringe!
Sucralose is made by chlorinating sugar, that is replacing 3 of the 8 hydroxyl (OH) groups with chlorine atoms. [Ed: Yes, I know, chlorine gas, Cl2 is poisonous, but this doens't mean that anything contains a chlorine atom is a poison, despite the claims here. But that's another post!] Such a substitution can utterly change the properties of the molecule, including it's taste. For example, replacing the OH group on ethanol (the alcohol we drink) produces an effective refrigerant (it's used as a local anesthetic, in fact), but not a good drink! An even smaller change, the inverting of two groups on the molecule that makes up spearmint oil, changes it into caraway oil (and you certainly would never say that mint tea tastes like rye bread). Bottom line, there is no reason that any given derivative of sugar will taste anything like sugar!
Much is actually known about the molecular characteristics necessary for sweetness.
Sucralose is made by chlorinating sugar, that is replacing 3 of the 8 hydroxyl (OH) groups with chlorine atoms. [Ed: Yes, I know, chlorine gas, Cl2 is poisonous, but this doens't mean that anything contains a chlorine atom is a poison, despite the claims here. But that's another post!] Such a substitution can utterly change the properties of the molecule, including it's taste. For example, replacing the OH group on ethanol (the alcohol we drink) produces an effective refrigerant (it's used as a local anesthetic, in fact), but not a good drink! An even smaller change, the inverting of two groups on the molecule that makes up spearmint oil, changes it into caraway oil (and you certainly would never say that mint tea tastes like rye bread). Bottom line, there is no reason that any given derivative of sugar will taste anything like sugar!
Much is actually known about the molecular characteristics necessary for sweetness.
Weird words of science 4
statins Anyone who reads the newspaper, listens to the news or watches TV will clearly associate the word statin with cholesterol, but in fact the source of the common name of this class of drugs comes from another biochemical pathway entirely. The suffix -statin was coined in 1973 in an article in Science by Brazeau: "We propose to name the peptide described here somatostatin, from somato(tropin), a pituitary factor affecting statural growth, and stat(in), from the Latin 'to halt, to arrest'." The cholesterol stopping statins were isolated around the same time (there are trends in scientific names, just as there are in baby names) and made use of the new suffix. A quarter century later in common use we've forgotten that there are other statins, such as somatostatin and nystatin, with no structural or theraputic relationship to the profitable cholesterol lowering agents.
For an intersesting take on trends in baby names, see Freakonomics, serialized here at Slate.
For an intersesting take on trends in baby names, see Freakonomics, serialized here at Slate.
Crestor, grapefruits and Italian towns have what in common?
In the current issue of the journal Circulation, there is a study supporting concerns that Crestor (known to chemists as rosuvastatin) is riskier than other statins. When you take most statins you can't drink grapefruit juice, which sounds like an odd prohibition, but for which there is a biochemical basis. Crestor is unusual, in that grapefruit juice does not affect the metabolism of the drug. So what is it in grapefruit juice that mucks up the behaviors of the statins?
Imbibing grapefruit juice (but not orange juice) raises the blood levels of the statins, making them more potent in terms of lowering cholesterol, but also more toxic. A component of the grapefruit juice apparently inhibits an enzyme responsible for the breakdown of the statins in the liver. One possible culprit is bergamottin.
If you drink Earl Grey tea, scented with oil of bergamot, this name may seem familiar to you. Etymologically, bergamottin is derived from the same source as bergamot, both stem from a citrus tree Citrus Bergamia , named for a town in Italy, Bergamo, where such trees presumably grow.
The cholesterol-lowering statins were first isolated from molds. The first (Lovastatin aka Mevacor) was isolated in the 70s from Aspergillus terreus .
Imbibing grapefruit juice (but not orange juice) raises the blood levels of the statins, making them more potent in terms of lowering cholesterol, but also more toxic. A component of the grapefruit juice apparently inhibits an enzyme responsible for the breakdown of the statins in the liver. One possible culprit is bergamottin.
If you drink Earl Grey tea, scented with oil of bergamot, this name may seem familiar to you. Etymologically, bergamottin is derived from the same source as bergamot, both stem from a citrus tree Citrus Bergamia , named for a town in Italy, Bergamo, where such trees presumably grow.
The cholesterol-lowering statins were first isolated from molds. The first (Lovastatin aka Mevacor) was isolated in the 70s from Aspergillus terreus .
Better Breathing Through Chemistry
Dr. Andy has posted about the marketing of an asthma control assessment by Glaxo-Smith-Kline (who not coincidently markets Advair as a treatment for asthma). I haven't seen any of the commercials, but I fairly sure my 8 year old has. He showed up in the kitchen last week and asked if he should take a test about his asthma and take it to his doctor. His asthma is mild and well controlled and I simply reminded him that if he had questions about it, he could ask at his next check-up. I wondered where he got the idea about the test, and since I don't watch TV, it took Dr. Andy's blog to point me in the right direction. The marketing is effective.
Last night sleep here was interrupted, in fact, by an asthma attack. Things resolved well using albuterol, but at 3 am I couldn't help wondering what I would have done 100 years ago, besides worry. Turns out inhalation devices for the treatment of asthma have been around since the 19th century at least. See examples here. Albuterol, a β-agonist, dates to the 1970s and is the most commonly used inhaled agent in its class in the United States. On the molecular level, it activates the β-2 receptor on the muscles surrounding the airways, relaxing them. It is fast acting, which was certainly a benefit last night. The structure is relatively simple.
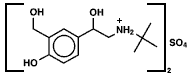
The molecule is chiral and albuterol is marketed both as a single stereoisomer and (most commonly) as the racemic mixture.
Last night sleep here was interrupted, in fact, by an asthma attack. Things resolved well using albuterol, but at 3 am I couldn't help wondering what I would have done 100 years ago, besides worry. Turns out inhalation devices for the treatment of asthma have been around since the 19th century at least. See examples here. Albuterol, a β-agonist, dates to the 1970s and is the most commonly used inhaled agent in its class in the United States. On the molecular level, it activates the β-2 receptor on the muscles surrounding the airways, relaxing them. It is fast acting, which was certainly a benefit last night. The structure is relatively simple.

The molecule is chiral and albuterol is marketed both as a single stereoisomer and (most commonly) as the racemic mixture.
I Wish I'd Made You Angry Earlier
Yesterday was Max Perutz's 101st birthday. Perutz won the Nobel in 1962 for his work in x-ray crystallography. I recently found a collection of essays he'd written (I Wish I'd Made You Angry Earlier: Essays on Science, Scientists, and Humanity and given the family history (both of my husband's parents were crystallographers of some note) picked it up to read.
The title essay is about Perutz's graduate research, where he and a colleague come close to unlocking the secret of the α-helix. Two things struck me in this essay. First was the origin of the terms α-helix and β-sheet. Bill Astbury, a crystallographer working with the Wool Research Associate in England had taken two crystal structures of a sample of kertain. The first diffraction experiment (the α sample) showed a simple and characteristic diffraction pattern, the β experiment, done after heating and stretching the sample gave a different pattern. Astbury concluded that the first pattern must arise from a coiled structure, the second from straight strands of amino acids laid out in a repeating pattern. Thus, theubiquitous α-helix and β sheet.
Perutz and Kendrew tried to crack the problem of figuring out just how the amino acids wound into the coil by building a model using a broomstick with nails hammered into to indicate the repeat (5.1 A). Even with all the sophisticated computer visualization (literally) at my fingertips, there is something about a tangible model that beats it all, even if I end up resorting (as I have) to using chickenwire. A few years ago, I solved a structural mystery by making a paper-doll like model of the molecule of interest.
The title? Pauling and Corey solved the mystery of the α-helix before Perutz and Kendrew. Reading the paper so angered and frustrated Perutz that he was able to design and execute the crucial experiment that proved beyond a doubt that Pauling and Corey were correct. Bragg, Pertuz's Ph.D. advisor told him that he'd wished he'd make him angrier earlier!
The title essay is about Perutz's graduate research, where he and a colleague come close to unlocking the secret of the α-helix. Two things struck me in this essay. First was the origin of the terms α-helix and β-sheet. Bill Astbury, a crystallographer working with the Wool Research Associate in England had taken two crystal structures of a sample of kertain. The first diffraction experiment (the α sample) showed a simple and characteristic diffraction pattern, the β experiment, done after heating and stretching the sample gave a different pattern. Astbury concluded that the first pattern must arise from a coiled structure, the second from straight strands of amino acids laid out in a repeating pattern. Thus, theubiquitous α-helix and β sheet.
Perutz and Kendrew tried to crack the problem of figuring out just how the amino acids wound into the coil by building a model using a broomstick with nails hammered into to indicate the repeat (5.1 A). Even with all the sophisticated computer visualization (literally) at my fingertips, there is something about a tangible model that beats it all, even if I end up resorting (as I have) to using chickenwire. A few years ago, I solved a structural mystery by making a paper-doll like model of the molecule of interest.
The title? Pauling and Corey solved the mystery of the α-helix before Perutz and Kendrew. Reading the paper so angered and frustrated Perutz that he was able to design and execute the crucial experiment that proved beyond a doubt that Pauling and Corey were correct. Bragg, Pertuz's Ph.D. advisor told him that he'd wished he'd make him angrier earlier!
Philatelic takes on famous scientists of this era (and others)
Earlier this month the USPS released a new set of stamps honoring four scientists: "some of the greatest scientists of our time, their pioneering discoveries still influence our lives today," according to John F. Walsh of the U.S. Postal Service's Board of Governors. Well, maybe! The four scientists are Barbara McClintock (geneticist), Richard Feynman (physicist), Josiah Willard Gibbs (thermodynamicist) and John von Neumann (mathematician/computer scientist). McClintock, Feynman and von Neumann are all more or less our contemporaries (their careers covered much of the last century)-- but Gibbs has been dead more than 100 years and I certainly would not count him "of our time".
Gibbs' name is familiair to almost any chemistry student - through the Gibbs free energy. J. Willard Gibbs (1839-1903) was the son of a Yale professor of sacred scripture, and himself worked at Yale. Gibbs was not paid a salary for the first 9 years of his job at Yale. It was only once he had a job offer from Johns Hopkins University in Maryland that Yale began to pay him. He gained little recognition for his work during his lifetime mainly because of his inability to communicate his ideas so that others could understand the concepts he was discussing.
With thanks to Tony Addison of Drexel for pointing me to the stamps.

Gibbs' name is familiair to almost any chemistry student - through the Gibbs free energy. J. Willard Gibbs (1839-1903) was the son of a Yale professor of sacred scripture, and himself worked at Yale. Gibbs was not paid a salary for the first 9 years of his job at Yale. It was only once he had a job offer from Johns Hopkins University in Maryland that Yale began to pay him. He gained little recognition for his work during his lifetime mainly because of his inability to communicate his ideas so that others could understand the concepts he was discussing.
With thanks to Tony Addison of Drexel for pointing me to the stamps.

Multiple Personalities 1: Wrists and snow
Decoding the eponyms of science and medicine offers a quick history lesson. The same names sometime surface in multiple contexts. Some are Renaissance men, others come out of scientific dynasties (think the Thompsons) , still others are just cases of "mistaken identity" (Fischer projections and Fischer carbenes).
Today is the birthday of Marcel De Quervain, a Swiss geologist who has done significant work on the physical properties of snow. A recent paper on the application of manure (really!) to snow and its effect on the melting of the ice pack refers to De Quervain's work.
If you have wrist problems, you may have heard of Fritz De Quervain who in 1895 described the tenosynovitis that bears his name.
Any relation? Not to my knowledge. Connections? You can aggravate your De Quervain's tenosynovitis if you ski...in the snow.
Today is the birthday of Marcel De Quervain, a Swiss geologist who has done significant work on the physical properties of snow. A recent paper on the application of manure (really!) to snow and its effect on the melting of the ice pack refers to De Quervain's work.
If you have wrist problems, you may have heard of Fritz De Quervain who in 1895 described the tenosynovitis that bears his name.
Any relation? Not to my knowledge. Connections? You can aggravate your De Quervain's tenosynovitis if you ski...in the snow.
The Invisible College
I spent part of today preparing for a talk for I'm giving at Drexel on Wednesday, for their E-Learning Lecture Series. Jean-Claude Bradley (whose lecture is linked to the posts on chirality) is my host. He's been constructing on-line courses in chemistry, that are also taught in real-time. By the end of term, most of the students are not present in the classroom, but are invisible in some sense to the lecturer. The web allows us to construct an invisible university, where neither chronological nor spatial constraints apply to the community of scholars.
This is nothing new. In the 17th centure, Robert Boyle, whose name we associate with the inverse relationship between pressure and volume, was part of an institutuion known as the Invisible College. The Invisible College was group of natural philosophers working in England, which Boyle joined in the 1650s. This group eventually became the Royal Society of London for Improving Natural Knowledge, still operating nearly 400 years later.
Interesting tidbits about Boyle: He identified himself as an alchemist and believed that base metals (such as iron) could be "transmuted" into more precious metals such as gold. The study of the properties of gases is the precursor of "scientific chemistry", and was an active field in the 17th century (think balloons!). Even though general chemistry books refer to Boyle's Law, it is also attributed in some texts (principally in Europe) to Mariotte. Boyle authored The Skeptical Chemist, where he encouraged experimentation and observation, and Some Considerations Touching the Usefulnesse of Experimental Natural Philosophy, where he strongly supported the teaching of experimental science in schools (if you don't enjoy lab, blame Boyle).
This is nothing new. In the 17th centure, Robert Boyle, whose name we associate with the inverse relationship between pressure and volume, was part of an institutuion known as the Invisible College. The Invisible College was group of natural philosophers working in England, which Boyle joined in the 1650s. This group eventually became the Royal Society of London for Improving Natural Knowledge, still operating nearly 400 years later.
Interesting tidbits about Boyle: He identified himself as an alchemist and believed that base metals (such as iron) could be "transmuted" into more precious metals such as gold. The study of the properties of gases is the precursor of "scientific chemistry", and was an active field in the 17th century (think balloons!). Even though general chemistry books refer to Boyle's Law, it is also attributed in some texts (principally in Europe) to Mariotte. Boyle authored The Skeptical Chemist, where he encouraged experimentation and observation, and Some Considerations Touching the Usefulnesse of Experimental Natural Philosophy, where he strongly supported the teaching of experimental science in schools (if you don't enjoy lab, blame Boyle).
Weird Words of Science 3
quantophrenic
A term used for an obsession with and exaggerated reliance upon mathematical methods or results. (Source Oxford English Dictionary). For a long time, chemists considered quantum theorists (of which I am one) to be quantophrenics. The following quote summed it up well: "Every attempt to employ mathematical methods in the study of chemical questions must be considered profoundly irrational and contrary to the spirit of chemistry. If mathematical analysis should ever hold a prominent place in chemistry - an aberration which is happily almost impossible - it would occasion a rapid and widespread degeneration of that science." Auguste Comte, Cours de Philosophie Positive, 1830. Fortunately, quantum chemists persisted, and the methods they developed to treat chemical systems have become powerful tools for chemists in many areas.
A term used for an obsession with and exaggerated reliance upon mathematical methods or results. (Source Oxford English Dictionary). For a long time, chemists considered quantum theorists (of which I am one) to be quantophrenics. The following quote summed it up well: "Every attempt to employ mathematical methods in the study of chemical questions must be considered profoundly irrational and contrary to the spirit of chemistry. If mathematical analysis should ever hold a prominent place in chemistry - an aberration which is happily almost impossible - it would occasion a rapid and widespread degeneration of that science." Auguste Comte, Cours de Philosophie Positive, 1830. Fortunately, quantum chemists persisted, and the methods they developed to treat chemical systems have become powerful tools for chemists in many areas.
Science in the Kitchen 2: Jello lasers
Last year my physical chemistry students and I attempted to design and build a dye laser from scratch. This is both easier and harder than you think. Several papers and an excellent web site provide background information and plans, but a good deal of patience is required to achieve an actual laser pulse. Limited time led to limited success for us.
Lots of different dyes will lase and you see the occasional reference on the web to Jello and whiskey lasers. These sound like urban legends, but in fact are not. Arthur Schawlow, who shared the Nobel Prize in physics in 1981 with Townes for building the laser, shows you can make Knox gelatin lase. See "Laser Action of Dyes in Gelatin", T. W. Hansch, M. Pernier, A. L. Schawlow, IEEE Journal of Quantum Electronics, January 1971, pp. 45-6. Interestingly, the longevity of the laser improves if you jiggle it.
Science in the Kitchen 1: Extracting DNA
Lots of different dyes will lase and you see the occasional reference on the web to Jello and whiskey lasers. These sound like urban legends, but in fact are not. Arthur Schawlow, who shared the Nobel Prize in physics in 1981 with Townes for building the laser, shows you can make Knox gelatin lase. See "Laser Action of Dyes in Gelatin", T. W. Hansch, M. Pernier, A. L. Schawlow, IEEE Journal of Quantum Electronics, January 1971, pp. 45-6. Interestingly, the longevity of the laser improves if you jiggle it.
Science in the Kitchen 1: Extracting DNA
Weird Words of Science 2
bra
A term coined by Dirac in 1947 to describe a vector in a function space that represents a wavefunction's complex conjugate. Kets are the notation for the corresponding vector representing a wavefunction. The words come from splitting "bracket", since when combined, the notation for looks like a bracket. "We shall call the new vectors bra vectors, or simply bras, and denote a general one of them by the symbol < |, the mirror image of the symbol for a ket vector." P. A. M. Dirac in "Principles of Quantum Mechanics"
I've been working on a paper today, and included an equation using bra and ket notation (which I find generally easier to follow than the intergral notation), which is what brought this to mind -- either that or it was the laundry I was folding!
A term coined by Dirac in 1947 to describe a vector in a function space that represents a wavefunction's complex conjugate. Kets are the notation for the corresponding vector representing a wavefunction. The words come from splitting "bracket", since when combined, the notation for looks like a bracket. "We shall call the new vectors bra vectors, or simply bras, and denote a general one of them by the symbol < |, the mirror image of the symbol for a ket vector." P. A. M. Dirac in "Principles of Quantum Mechanics"
I've been working on a paper today, and included an equation using bra and ket notation (which I find generally easier to follow than the intergral notation), which is what brought this to mind -- either that or it was the laundry I was folding!
Sweet secrets
No one wants to run the cotton candy machine at the school fair, and now I know why. The plant booth is serene under the trees, the bake sale treats are neatly packaged up, even the lemon mom has only sticky hands. I am covered in pink, blue and violet spun sugar from head to foot. Really, I was seeing the world through rose colored glasses. And it gave me a lot of time to muse about sugar -- why is it so sticky?
What my recipe book calls sugar a chemist calls sucrose (or in very formal situations: [beta]-D-fructofuranosyl [alpha]-D-glucopyranoside). The stickyness of sugar is related to its hygroscopic nature (it will pick up water out of the air), which is a function of its molecular structure (for example the polar nature of the groups on the surface of the molecule - lots of OH groups.) and the physical form of the sugar (why cotton candy noticeably more hygroscopic than granular table sugar).
Biologically, the stickyness of sugar has certain advantages, as an article in Journal of Molecular Biology last year suggests.
And yeah - I'll do the cotton candy again next year!
What my recipe book calls sugar a chemist calls sucrose (or in very formal situations: [beta]-D-fructofuranosyl [alpha]-D-glucopyranoside). The stickyness of sugar is related to its hygroscopic nature (it will pick up water out of the air), which is a function of its molecular structure (for example the polar nature of the groups on the surface of the molecule - lots of OH groups.) and the physical form of the sugar (why cotton candy noticeably more hygroscopic than granular table sugar).
Biologically, the stickyness of sugar has certain advantages, as an article in Journal of Molecular Biology last year suggests.
And yeah - I'll do the cotton candy again next year!
Weird Words of Science 1
virial
The virial equation provides both a general form for an equation of state for gases or liquids, as well as a connection (through its coefficients) to the microscopic level intermolecular forces experienced by the atoms and molecules. The equation of state can be used (along with other information) to arrive at a phase diagram. The Latin root of the word is vir which is a plural form of vis or force.
Physical chemists are quite fond of the virial expansion, as this quote might suggest:
The virial equation provides both a general form for an equation of state for gases or liquids, as well as a connection (through its coefficients) to the microscopic level intermolecular forces experienced by the atoms and molecules. The equation of state can be used (along with other information) to arrive at a phase diagram. The Latin root of the word is vir which is a plural form of vis or force.
Physical chemists are quite fond of the virial expansion, as this quote might suggest:
"The virial expansion is one of the cleanest-cut developments in the subject of statistical mechnics." Condon and Odishaw in the Handbook of Physics, 1967.
Thermodynamics of Spring
Rumor on the playground this evening was that the weather is warming (they're predicting 80F for Monday!). As Art Carey's recent article in the Philadelphia Inquirer points out, water temperatures will lag behind. Chuck Sutherland, an avid sea-kayaker, points out the perils of cold water. The article notes that cold water will lower your body temperature 25 times faster than cold air, as anyone who's ever jumped in a cool pool can attest!
So, why is cold water "colder" than cold air (at the same apparent temperature)?
A little statistical thermodynamics is a help here. What we sense on the macroscopic level as heat, at the microscopic level is the result of molecular motion. The faster a herd of molecules moves, the "hotter" they are. To move energy from one set of molecules (say, those in your skin) to another (those in the water) requires a collision. This is why a thermos works, by making a near vaccum, where there are very few molecules to collide, you reduce heat transfer. The more collisions, the more heat gets transfered. The density of molecules in liquid water is about 1000 times higher than that in air, leading to more collisions in any given period of time and so increased heat transfer.
So, why is cold water "colder" than cold air (at the same apparent temperature)?
A little statistical thermodynamics is a help here. What we sense on the macroscopic level as heat, at the microscopic level is the result of molecular motion. The faster a herd of molecules moves, the "hotter" they are. To move energy from one set of molecules (say, those in your skin) to another (those in the water) requires a collision. This is why a thermos works, by making a near vaccum, where there are very few molecules to collide, you reduce heat transfer. The more collisions, the more heat gets transfered. The density of molecules in liquid water is about 1000 times higher than that in air, leading to more collisions in any given period of time and so increased heat transfer.
The Culture of Science: The Tangled Bank
Visit The Tangled Bank, an eclectic bi-weekly guide to science writing on the web. Buridan's Ass is this week's host. My lateral take on left versus right is highlighted there and can be found at One Purple Pill is Much Like Another and How Old is a Whale? below.
If you've ever wondered what an epicycle is, visit the Canadian Cynic to find out. (Though if you played with a Spirograph as a kid, turns out you already know what an epicycle is!). Orac Knows' field guide to poster sessions is a great read as well! Check out the Tangled Bank to discover lots more...
If you've ever wondered what an epicycle is, visit the Canadian Cynic to find out. (Though if you played with a Spirograph as a kid, turns out you already know what an epicycle is!). Orac Knows' field guide to poster sessions is a great read as well! Check out the Tangled Bank to discover lots more...
Measuring height with a thermometer
Phase diagrams can be plot devices in more than one sense. In the mid-nineteenth century, the British empire was anxious to map the lands north of India. The British and the Russian were engaged in a "Great Game", in which geographical information was but one of the prizes, as each sought to expand their area of control in Asia. Unfortunately for the British, the emperor of China had closed the borders with India to foreigners, under penalty of death. More than one British surveyor perished in attempts to cross the border and map Tibet. Eventually Thomas Montgomerie of the British Survey hit upon recruiting well-educated Indians as clandestine surveyors whose cover identity would be that of itinerant lamas.
The "pundits" were given extensive training over two years and learned to use a sextant, measure altitude by boiling point determination (This is an application of the Clausius-Clapeyron equation: as altitude increases, atmospheric pressure decreases and the boiling point of water decreases in a known relationship) and to walk a measured pace. Pundits counted their paces with the traditional rosary carried by holy men; their rosaries had been altered to contain only 100 beads instead of the usual 108 and a single circuit of the beads was equivalent to a traverse of 5 miles. Observations were recorded on the papers inserted into their prayer wheels.
One of the first pundits was Nain Singh, who at 33 and headmaster of a school in the Himalayas was recruited along with his cousin Mani Singh to survey Tibet. Singh walked thousands of miles in his mapping ventures, and the maps constructed from his survey were the best available until well into the 20th century. On his third and last survey journey, Singh traveled more than eight months from Leh to Lhasa to map the trade route. His measurement of the altitude of Lhasa using measurements of the boiling point of water were accurate to within a hundred meters. Singh retired after this journey to train other pundits. He was well recognized for his feats by many geographical societies and the government of India in his later years. If this all sounds vaguely familiar, it may be because Rudyard Kipling drew heavily on the events around the "Great Game" in his novel, Kim.
The "pundits" were given extensive training over two years and learned to use a sextant, measure altitude by boiling point determination (This is an application of the Clausius-Clapeyron equation: as altitude increases, atmospheric pressure decreases and the boiling point of water decreases in a known relationship) and to walk a measured pace. Pundits counted their paces with the traditional rosary carried by holy men; their rosaries had been altered to contain only 100 beads instead of the usual 108 and a single circuit of the beads was equivalent to a traverse of 5 miles. Observations were recorded on the papers inserted into their prayer wheels.
One of the first pundits was Nain Singh, who at 33 and headmaster of a school in the Himalayas was recruited along with his cousin Mani Singh to survey Tibet. Singh walked thousands of miles in his mapping ventures, and the maps constructed from his survey were the best available until well into the 20th century. On his third and last survey journey, Singh traveled more than eight months from Leh to Lhasa to map the trade route. His measurement of the altitude of Lhasa using measurements of the boiling point of water were accurate to within a hundred meters. Singh retired after this journey to train other pundits. He was well recognized for his feats by many geographical societies and the government of India in his later years. If this all sounds vaguely familiar, it may be because Rudyard Kipling drew heavily on the events around the "Great Game" in his novel, Kim.
Global Freezing
It's May and there is a chance of frost tonight in Philadelphia. It's been cold and damp here, which made me think of Kurt Vonnegut's novel, Cat's Cradle, where the whole world was in peril of freezing (or at least all the water).
In the novel Vonnegut proposes a new solid form of water, Ice IX, which is more stable that liquid water at "normal" pressures and temperatures. Once released into the world at large, it would catalyze the conversion of all liquid water to this solid form - permanently! Some analyses of the text suggest that Vonnegut's brother (who had a Ph.D. in physical chemistry from MIT) might have seeded this idea. Ice IX was not actually discovered until 1968, about 5 years after Cat's Cradle appeared (and doesn't catalyze the conversion of liquid water to a solid form).
We tend to think of only 3 phases of water: ice, liquid water and steam, but there are many forms of ice, each with a particular structure. Ice IX is stable at high pressures (several hundred atmospheres) and low temperatures. The pressures and temperatures of various forms of ice are summarized in a phase diagram here.
Hal Clement had a story called "Phases in Chaos" where the protagonist relied on his recall of the phase diagram of water to negotiate a complex extraterrestial undersea environment. You never know when you'll need to know a bit of physical chemistry!
In the novel Vonnegut proposes a new solid form of water, Ice IX, which is more stable that liquid water at "normal" pressures and temperatures. Once released into the world at large, it would catalyze the conversion of all liquid water to this solid form - permanently! Some analyses of the text suggest that Vonnegut's brother (who had a Ph.D. in physical chemistry from MIT) might have seeded this idea. Ice IX was not actually discovered until 1968, about 5 years after Cat's Cradle appeared (and doesn't catalyze the conversion of liquid water to a solid form).
We tend to think of only 3 phases of water: ice, liquid water and steam, but there are many forms of ice, each with a particular structure. Ice IX is stable at high pressures (several hundred atmospheres) and low temperatures. The pressures and temperatures of various forms of ice are summarized in a phase diagram here.
Hal Clement had a story called "Phases in Chaos" where the protagonist relied on his recall of the phase diagram of water to negotiate a complex extraterrestial undersea environment. You never know when you'll need to know a bit of physical chemistry!
How old is a whale? Chirality has a clue...
Helis the whale is summering in Philadlephia. Spotted a couple of weeks ago as far north in the Delaware as Trenton, and then again this week in the Schuylkill River, biologists speculate that Helis has come to enjoy the warm (relatively speaking) waters in his old age. Beluga whales typically range much further north than the Delaware Bay, and Helis comes from the St. Lawrence River estuary. He has been observed there since 1986, which means he must be at least 20 years-old, but is probably closer to 30 years old and nearing the end of his life. How do you tell how old a whale is? How long do they live? Humans are among the longest-living mammals, with a life-span on the order of 100 years. Human ages can be verified by consulting birth and census records. Whales are also apparently very long-lived, but discovering the age of a whale is a somewhat more daunting task and the life-spans of most whale species have not been established.
In the case of belugas like Helis, longitudinal observations of populations provide an answer. Individual whales are identified, then followed for their lifetimes, the cetacean equivalent of the US Census. Many whale populations are not regulars in rivers, and not so easily followed -- what then? The concept of chirality again comes into play as chemists and biologists try to answer the question.
The ages of some whales, such as blue whales and fin whales can be determined by counting the layers of ear wax in their inner ears. [Really!] These whales have an apparent life-span similar to that of humans, between 80 and 100 years. Bowhead whales, which live north of the Artic circle, are still hunted in limited numbers by the Inuit. Since the early 1980s, several clearly ancient harpoon heads have been found embedded in modern bowhead whales. The types of points recovered had not been used since the late 19th century, suggesting the whales were more than 100 years old. Inuit oral histories also supported a long life span for these whales, as multiple generations of hunters described encountering the same whale.
There are two forms of the amino acid aspartic acid, R and S. Amino acids are the building blocks of proteins. The S form os aspartic acid is what is found in naturally occuring proteins. Over time, some of the S form will turn into the R form, a process called racemization. The degree of racemization of aspartic acid in the lense of the eye has been used to find the ages in 20 different species, including humans. Ages were obtained for 48 different whales, one which had an apparent age of 211 years [Can. J. Zool. 1999, 77, 571-580], making the bowheads the longest living mammals known!
In the case of belugas like Helis, longitudinal observations of populations provide an answer. Individual whales are identified, then followed for their lifetimes, the cetacean equivalent of the US Census. Many whale populations are not regulars in rivers, and not so easily followed -- what then? The concept of chirality again comes into play as chemists and biologists try to answer the question.
The ages of some whales, such as blue whales and fin whales can be determined by counting the layers of ear wax in their inner ears. [Really!] These whales have an apparent life-span similar to that of humans, between 80 and 100 years. Bowhead whales, which live north of the Artic circle, are still hunted in limited numbers by the Inuit. Since the early 1980s, several clearly ancient harpoon heads have been found embedded in modern bowhead whales. The types of points recovered had not been used since the late 19th century, suggesting the whales were more than 100 years old. Inuit oral histories also supported a long life span for these whales, as multiple generations of hunters described encountering the same whale.
There are two forms of the amino acid aspartic acid, R and S. Amino acids are the building blocks of proteins. The S form os aspartic acid is what is found in naturally occuring proteins. Over time, some of the S form will turn into the R form, a process called racemization. The degree of racemization of aspartic acid in the lense of the eye has been used to find the ages in 20 different species, including humans. Ages were obtained for 48 different whales, one which had an apparent age of 211 years [Can. J. Zool. 1999, 77, 571-580], making the bowheads the longest living mammals known!
Subscribe to:
Comments (Atom)















